|
|
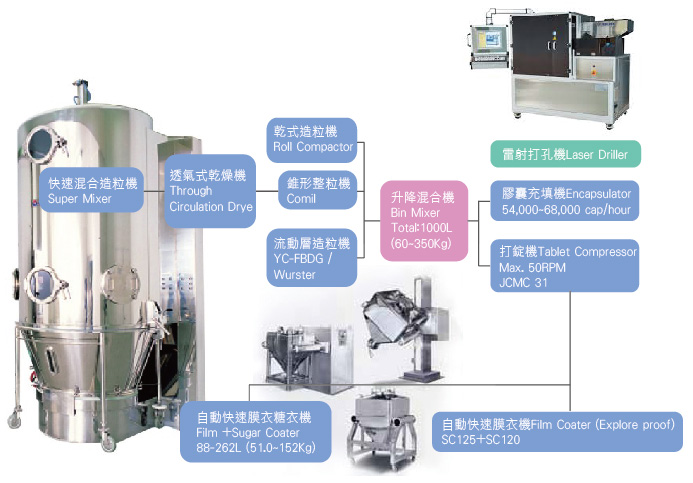
Our company is a professional pharmaceutical manufacturing plant, specializing in various dosage forms including solid dosage forms (such as tablets, film-coated tablets, sugar-coated tablets, sustained-release tablets, granules, and powders), sustained-release pellets in capsules (including general capsules), pellets, suspensions (including solutions), and so on. We are also equipped with a variety of equipment for producing various dosage products. This includes different types of high-shear granulation machines with top or bottom blades, Glatt fluidized bed with top spray granulation, Wurster’s bottom spray coating technology, Freund’s Granurex pellet side-spray coating machine, and constant-pressure roller compactor (dry granulator). All of these ensure stable manufacturing to meet the diverse requirements of various granulation technology platforms. Besides, AUPA is equipped with a number of tablet press equipment, including double-layer and triple-layer tablet press machines, to manufacture and monitor different drug layers. We have not only autonomously developed our own technology but also recruited talents with extensive manufacturing and technology transfer experience from leading international GMP pharmaceutical manufacturers. Their participation forms our core technical service team, ensuring our excellent problem-solving capabilities and smooth technology transfer processes from clinical to commercial production. We also provide bottling and PTP packaging services. Our packaging equipment is capable of the international GS1 barcode packaging system, which is rare in domestic and aligned with international pharmaceutical standard. It includes functionality for serialization and post-sales traceability of product manufacturing information, providing added safety and assurance in medication use. |